ocrevus start up form
These infusion reactions can happen for up to 24 hours after your infusion. Tell your caregiver if you feel dizzy.
![]()
Ocrevus Connects Patient Support Program
Review TYSABRIs Well-Undesrtood Safety Profile.

. 2 Patient Authorization and Additional Consents. In the beginning I had brain fog and fatigue. View Doctor Resources Downloadable Start Forms.
According to her the only one that is consistently. Prescribers first name. The OCREVUS Start Form is required for enrollment in OCREVUS Access Solutions.
Ocrevus may cause unpleasant side effects. These infusion reactions can happen for up to 24 hours after your infusion. Ad Learn More About OCREVUS Resources And How Patient Navigators Can Help.
See Website For Safety Boxed Warning PI. Once youve written a prescription for OCREVUS complete the Start Form or enroll patients online to get them started with OCREVUS. 300mg in 250ml 09 sodium chloride Frequency.
Complete entire form and fax to Alongside KESIMPTA at 1-833-318-0680 An incomplete Start Form may delay the start of treatment. Have your patient read the. O Start dose300 mg intravenous infusion followed two weeks later by a second 300 mg intravenous infusion 23 o Subsequent doses.
Discover Why More People Are Choosing To Start With Or Switch To OCREVUS. It is a one-time registration completed by the practice. Prescription Enrollment Form.
Access PI and Start Resources for HCPs. Ocrevus Order Form Prescriber Signature Date Please Print Name Form 350 NForms300 - PHARMACYF350 - Ocrevus Physician Order Formdocx Orders are initiated unless crossed out. Fax completed form to.
Relapsing forms of multiple sclerosis MS to include clinically isolated syndrome relapsing-remitting disease and. Discover Why More People Are Choosing To Start With Or Switch To OCREVUS. Deciding If TYSABRI May Be An Option.
Start at 30mlhr increasing by 30mlhr every 30 minutes to a. During my second infusion appointment I asked the nurse what types of side effects she saw when people start Ocrevus. See Website For Safety Boxed Warning PI.
This form is used to initiate the EFT registration process when the practice chooses not to use check reimbursements. Ocrevus ocrelizumab injection is a preservative-free sterile clear or slightly opalescent and colorless to pale brown solution supplied as a carton containing one 300. View Doctor Resources Downloadable Start Forms.
Contact Us for Support. Ad Learn About KESIMPTA. Ocrevus intravenous infusion Induction.
On Day 1 and Day 15 Rate. Ocrevus is the number one prescribed MS medication in the US. The physician portion and submit this completed form.
When possible you should receive any non-live vaccines at least 2 weeks before you start treatment with. Ad Check Out The Efficacy Safety Of TYSABRI. Ocrevus ocrelizumab Fax completed form to 8883021028.
600mg intravenous infusionevery 6 months23. Ad View 5 Years of Safety Data - 2 Years Controlled 3 Years Open Label Extension. Reducing the Risk of.
When possible you should receive any non-live vaccines at least 2 weeks before you start. OCREVUS is a prescription medicine used to treat. Ad Get patients started with AUBAGIO.
Benefits investigation Benefits reverification approximately 6. I started Ocrevus last Oct 31 and am hold because as well as bringing my CD19 B cells to zero as is usual it took my t cells down drastically too both CD4s CD8s. By completing this form you are requesting services on behalf of your patient which may include.
If this is an URGENT request please call 800 882-4462. These infusion reactions can happen for up to 24 hours after your infusion. Deciding If TYSABRI May Be An Option.
Pamela Arterbridge kept busy with her family and her career as a hairstylist for 25 years but knew there was something not right. Ad View 5 Years of Safety Data - 2 Years Controlled 3 Years Open Label Extension. Start at 30 min at 60 min at 90 min at 120 min at 150 min 30 mLhr 60 mLhr 90 mLhr 120 mLhr 150 mLhr 180 mLhr Infusion rate tables1 INITIAL DOSE 600 MG ADMINISTERED AS 2.
OCREVUS Start Form Ready to get started. Swelling of the throat. Access PI and Start Resources for HCPs.
It must be completed by the provider. Ad Learn About KESIMPTA. Some side effects may occur during the injection or up to 24 hours later.
Ad Learn About KESIMPTA. OCREVUSTM is indicated for the treatment of adult patients with relapsing or primary. Contact Us for Support.
Plegridy Vumerity Zeposia Ocrevus. Ad Learn More About OCREVUS Resources And How Patient Navigators Can Help. Review TYSABRIs Well-Undesrtood Safety Profile.
Inform patients that infusion reactions can occur up to 24 hours after the infusion. The OCREVUS Start Form is required for enrollment in OCREVUS Access Solutions. Ad Check Out The Efficacy Safety Of TYSABRI.
The form includes patient insurance and prescription information. Patients first name. S this a new start or continuation of therapy.
Date of birth. For patients starting a new treatment and more than 200000 people have now been treated with Ocrevus.

Top 100 Medical Device Startups With Most Money Raised In 2020 Free Chart
Ocrevus Connects Patient Support Program
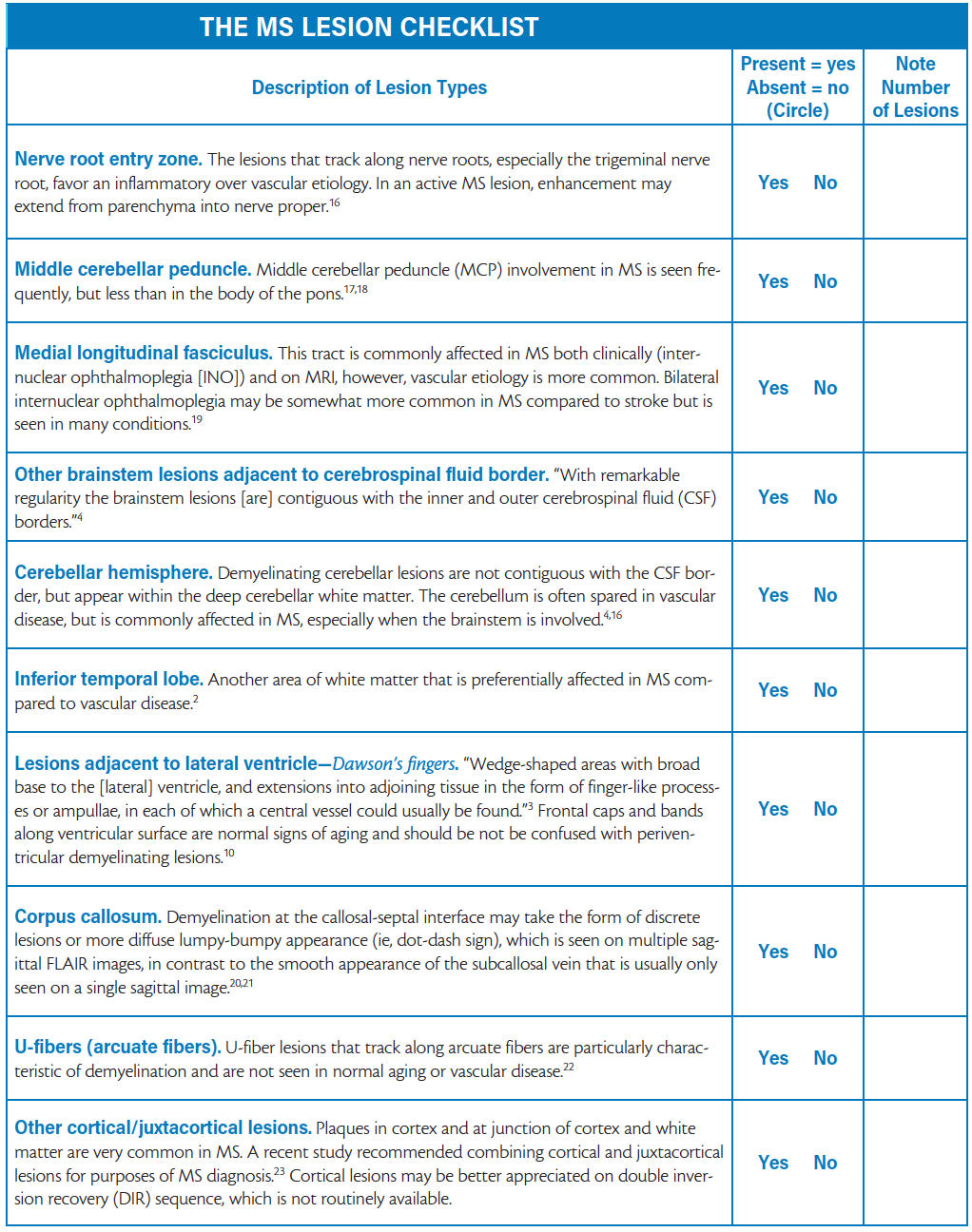
The Multiple Sclerosis Lesion Checklist Practical Neurology

Ocrevus Connects Patient Support Program

Ocrevus Side Effects Cost Uses And More

Chronically Ill Traumatically Billed The 123 000 Medicine For Ms Kaiser Health News

Ocrevus Connects Patient Support Program

Submit Print Or Download Ocrevus Forms Documents Ocrevus Access Solutions
![]()
Ocrevus Connects Patient Support Program
![]()
Ocrevus Connects Patient Support Program

Ocrevus Ocrelizumab Ms Infusion Experience

Ocrevus Connects Patient Support Program

Ocrevus Ocrelizumab Ms Infusion Experience

Submit Print Or Download Ocrevus Forms Documents Ocrevus Access Solutions

